
|
|||
|---|---|---|---|
|
|
Leucine stimulates mTOR and muscle protein synthesis in both animal and human |
|
|
|
Nutricionistas, Pós-graduandos pelo Programa de Pós-Graduação em Nutrição UNIFESP – São Paulo (Brasil) |
Gustavo Duarte Pimentel Juliane Costa Silva Zemdegs |
|
|
|
Abstract Several studies shows that leucine contributes to the activation of mammalian target of rapamycin (mTOR) signalling and to the inhibition of the adenosine monophosphate kinase (AMPK) activity, processes that potentially enhance protein synthesis and skeletal muscle growth. Keywords: Leucine. mTOR. Protein synthesis. |
|||
|
|
http://www.efdeportes.com/ Revista Digital - Buenos Aires - Año 14 - Nº 131 - Abril de 2009 |
|
|
1 / 1
Several studies have reported that leucine supplementation is an effective strategy to increase muscle protein synthesis (1-4) in both rodents(2,3) and humans (4).
The anabolic mechanism is performed by the activation of the mRNA translational machinery through the mammalian target of rapamycin (mTOR), a protein with a molecular weight of around 290 kD (5), in an insulin dependent or independent manner (6,7).
The mTOR signaling is activated by hormones and growth factors, e.g. insulin and insulin like growth factor (IGF-1), respectively. These anabolic factors activate the phosphatidyl inositol-3-phosphate kinase (PI3-k) pathway and protein kinase B (PKB/Akt) leading to mTOR phosphorylation (5,8).
Besides, leucine treatment also enhances the phosphorylation of mTOR, and its activation up-regulates protein translation through the phosphorylation of the eukaryotic initiation factor 4E binding protein 1 (4E-BP1) and the ribosomal protein S6 kinase (S6K) (4,9,10), leading to cell growth and proliferation.
Nevertheless, unlike insulin and IGF-1, leucine has a direct effect at an intracellular locus modulating protein signaling pathways. Besides, leucine do not appear to require the mediation of a cell membrane receptor (11), acting efficiently in the protein synthesis.
Leucine and other BCAA have a distinct metabolic pathway. BCAA are not degraded in the liver due to its absence of branched-chain amino acid aminotransferase (BCAT), while other amino acids are mainly metabolized in liver. Otherwise, BCAT is present in skeletal muscle (12) and degradation of BCAA in the skeletal muscle is hypothesized to provide energy which inhibits AMPK activity, leading to mTOR activation and the increase of protein synthesis.
Leucine intake increases ATP content in muscle cells and reduces the AMP/ATP ratio, confirming that leucine is used to generate energy in muscle cells. Some studies propose that leucine activates mTOR in part through the inhibition of the adenosine monophasphate protein kinase (AMPK). To put in another way, the AMPK activity is reduced during the leucine treatment and when AMPK is activated, the mTOR signalling is impaired (13).
In line with this, Gallagher et al (4) reported that the resistance exercise in combination with the intake of branched-chain amino acid (BCAA) activates the hypertrophic signaling in the skeletal muscle. Moreover, those authors suggest that the supplementation of BCAA is more effective than the resistance exercise to increase protein synthesis.
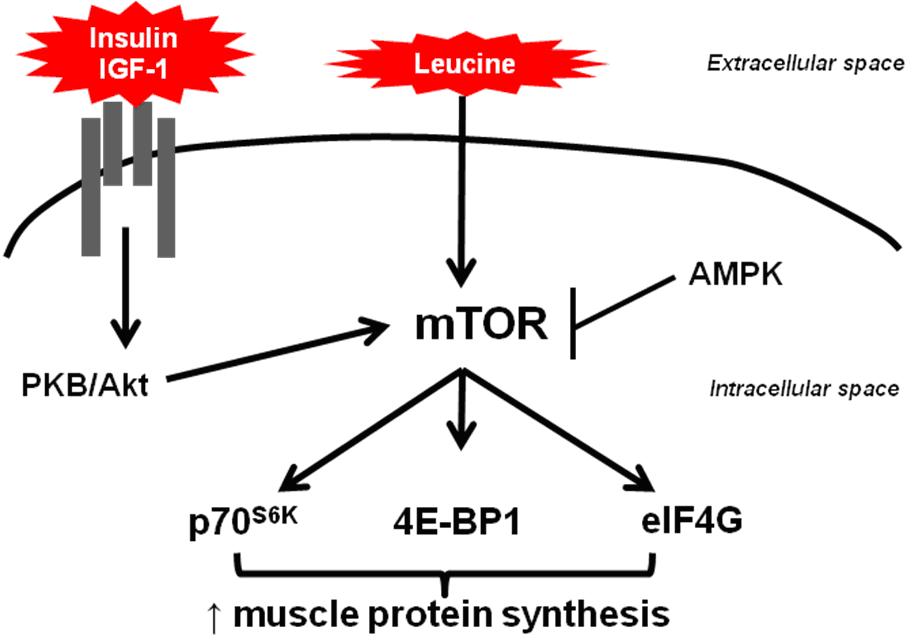
Figure 1. Activation of the mammalian target of rapamycin (mTOR) pathway in the muscle protein synthesis by leucine and anabolic factors.
IGF-1: insulin like growth factor, PKB/Akt: protein kinase B, AMPK: adenosine monophasphate protein kinase, mTOR: mammalian target of rapamycin,
p70S6K: ribosomal protein S6 kinase, 4E-BP1: eukaryotic initiation factor 4E binding protein 1, eIF4G: eukaryotic initiation factor 4G.
Figure 1 shows schematically the role of leucine, insulin and IGF-1 in the activation of the mTOR pathway in the muscle protein synthesis.
Conclusion
In summary, leucine stimulates mTOR signalling and protein synthesis in muscle cells, in part through inhibition of AMPK. It follows from this that nutritional management is a potential strategy to promote mTOR signalling and muscle protein synthesis in sarcopenic-lean, sarcopenic-obese, and senile sarcopenic individuals.
References
-
Koopman R, Wagenmakers AJ, Manders RJ, Zorenc AHG, Senden JMG, Gorselink M, et al. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab. 288(4):E645-53, 2005.
-
Anthony JC, Anthony TG, Layman DK. Leucine supplementation enhances skeletal muscle recovery in rats following exercise. J Nutr. 129(6):1102-6, 1999.
-
Anthony JC, Reiter AK, Anthony TG, Crozier SJ, Lang CH, MacLean DA, et al. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E-BP1 or S6K1 phosphorylation. Diabetes. 51(4):928-36, 2002.
-
Gallagher P, Richmond S, Dudley K, Prewitt M, Gandy N, Kudrna B, et al. Interaction of resistance exercise and BCAA supplementation on Akt and p70 s6 kinase phosphorylation in human skeletal muscle. FASEB J. 21:895.10, 2007 [Abstract].
-
Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr. 131(3):856S-860S, 2001.
-
Proud CG. mTOR-mediated regulation of translation factors by amino acids. Biochem Biophys Res Commun. 313(2):429-36, 2004.
-
Liu Z, Jahn LA, Long W, Fryburg DA, Wei L, Barrett EJ. Branched chain amino acids activate messenger ribonucleic acid translation regulatory proteins in human skeletal muscle, and glucocorticoids blunt this action. J Clin Endocrinol Metab. 86(5):2136-43, 2001.
-
Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology. 21(10):362-9, 2006.
-
Shen WH, Boyle DW, Wisniowski P, Bade A, Liechty EA. Insulin and IGF-I stimulate the formation of the eukaryotic initiation factor 4F complex and protein synthesis in C2C12 myotubes independent of availability of external amino acids. J Endocrinol. 185(2):275-89, 2005.
-
Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates mTOR action. Cell. 110(2):177-89, 2002.
-
Beugnet A, Tee AR, Taylor PM, Proud CG. Evidence that intracellular amino acids regulate translation factor function in mammalian cells. Biochem J. 372(1):555-66, 2002.
-
Sweatt AJ, Wood M, Suryawan A, Wallin R, Willingham MC, Hutson SM. Branched-chain amino acid catabolism: unique segregation of pathway enzymes in organ systems and peripheral nerves. Am J Physiol Endocrinol Metab. 286(1):E64-76, 2004.
-
Du M, Shen QW, Zhu MJ, Ford SP. Leucine stimulates mTOR signaling in C2C12 myoblasts in part throught inhibition of AMP-activated protein kinase. J Anim Sci. 85: 919-27, 2007.
Another articles In English
 |
|
|---|---|
|
revista
digital · Año 14 · N° 131 | Buenos Aires,
Abril de 2009 |
|